The 7 Colors of Hydrogen
Hydrogen is colourless – that is a well known fact and not only a definition from Encyclopædia Britannica, and yet, hydrogen is frequently referred to a having a specific colour. However, the colour coding does not define the actual optical appearance of the gas. Rather, it is used to indicate its ecological footprint and its carbon intensity in particular.

Occurrence and Production of Hydrogen
With more than 90% of all atoms and roughly 75% of matter, hydrogen is the most common chemical element in the universe. However, hydrogen only contributes to less than 1% of earth’s overall mass and hardly any terrestrial hydrogen is available as H2 but in more complex molecules like water or methane (i.e. natural gas), making it impossible to exploit natural hydrogen reservoirs on earth.
To make hydrogen available in large quantities one needs to split up molecules which are available in vast quantity. This is primarily done using three processes: steam reforming, methane pyrolysis, and electrolysis.
Today’s hydrogen production primarily relies on steam reforming. In steam reforming, a hydrocarbon – primarily methane – reacts with steam to hydrogen and carbon monoxide. In a subsequent process step, additional water is used to produce carbon dioxide and additional hydrogen from the carbon monoxide.
Another technology, methane pyrolysis, uses methane as starting point. The gas is thermally cracked into hydrogen and carbon. As carbon is a solid, it can easily be separated and stored or used in other processes, thus avoiding any carbon dioxide emissions to the atmosphere.
Perhaps the best-known process for hydrogen production probably is water electrolysis. As most terrestrial hydrogen can be found in water, this technology levers the largest hydrogen reservoir on earth. Electricity is used to split water into hydrogen and oxygen. As no carbonaceous molecules are involved in the process, it does not emit any carbon dioxide at all.

That is How Hydrogen Becomes Colourful
Hydrogen and its derivatives, i.e. energy carriers produced from hydrogen, are an important building block for a sustainable and climate neutral energy supply as no carbon dioxide is emitted when using hydrogen. For a sound evaluation of hydrogen’s climate impact, however, it is necessary to understand the way it is produced – and that is why its colour nomenclature makes sense.
The color of hydrogen determines both the primary hydrogen and energy carrier and the production process. This set of information allows for a rough estimation of the climate footprint of a specific batch of hydrogen and thus whether is can be considered climate friendly or not.
Unfortunately, different users are using different color codes which, in some details, differ from each other. We are following the definitions used by Germany’s National Hydrogen Council and the German Government.
Hydrogen produced from methane and other hydrocarbons using steam reforming is labeled as grey hydrogen. Assuming perfect process conditions, four hydrogen molecules and one carbon dioxide molecule are produced from one methane and two water molecules. The energetic efficiency of the process is at roughly 70%.
The carbon dioxide emissions from this process can be captured and permanently stored underground, either in gas caverns or suited geological formations. While the required process steps prevent emitting carbon dioxide in the atmosphere, they also reduce efficiency as additional energy is required for carbon sequestration and compression. Hydrogen produced by steam reforming from fossil hydrocarbons utilizing carbon capture is labeled as blue hydrogen.
Turquoise hydrogen is hydrogen produced by methane pyrolysis. As with grey and blue hydrogen, the production process utilizes fossil hydrocarbons. But since the byproduct is not carbon dioxide but solid carbon, carbon dioxide emissions are avoided if the energy required for the pyrolysis process is produced in a carbon neutral way. The technology is currently being developed but not yet commercially available at larger scale.
Red, yellow, and green hydrogen all stem from electricity using water electrolysis. There are no direct carbon dioxide emissions, and the energetic efficiency of commercial systems today is in the 60-65% ballpark. The different colors signify differnt sources of electricity.
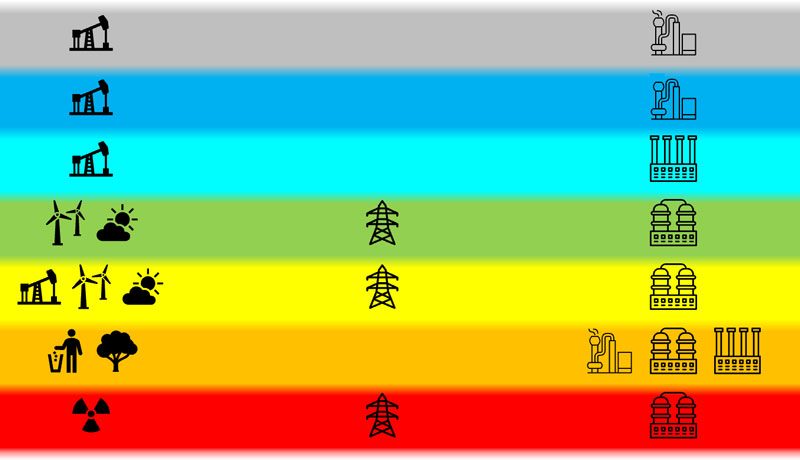
Yellow hydrogen is hydrogen made from grid power. The energy mix, i.e. the percentage of fossil, nuclear, and renewable energy, is given by the power generation at the very moment the hydrogen is produced. The measure of carbon dioxide emissions of yellow hydrogen are varying because the energy mix is constantly changing – due to availability in solar and wind power and changes on the demand side. In case of a high percentage of coal and gas in the energy mix, the effective emissions of yellow hydrogen may temporarily even be worse than those of grey hydrogen.
In case only electricity from nuclear power plants is used for hydrogen production, this hydrogen is labeled as red. No carbon dioxide is emitted in its entire production process and thus it is climate neutral. However, nuclear power generation produces radioactive wastes which need to be securely stored for centuries. Thus, red hydrogen comes with additional risks.
Electrolysis utilizing only electricity from renewable sources, i.e. wind solar and hydro power, produces so-called green hydrogen. In the process, neither carbon dioxide nor any other harmful substances are produced. Unfortunately, the availability of renewable electricity is generally limited by weather conditions. Therefore, for a continuous production of green hydrogen, large scale batteries are required for storage of electricity.
Finally, orange hydrogen summarizes all production pathways utilizing waste or biomass as input. It is, however, impossible to have a general statement on overall efficiency and cabron bioxide emissions as this will vary depending on the input and the respectively used production technology.
You like to learn more about how to set up a sustainable and economical hydrogen supply? Contact us or follow us on LinkedIn for regular updates.





