Green Hydrogen is considered key to a sustainable and climate neutral energy supply. Neither its production nor its consumption emits carbon dioxide, it can be stored and transported without significant losses and allows for sector coupling. However, these benefits com at a cost. For 2019, statista estimates the costs of the production for green hydrogen at 16.50 €/kg.
Key Drivers of Hydrogen Production Costs
The main obstacle to the commercial success of green hydrogen is its cost compared to other sources of energy. Therefore, one of the most important questions is: How can we reduce
Production costs? To better understand this question and its implications, let’s take a deep dive into what contributes to hydrogen’s production costs.
The main elements defining its production costs are:
- investment,
- fixed operational costs,
- variable operational costs and
- utilisation.
By investment is meant all expenditure related to hardware incl. engineering and erecting – from electrolysis to piping and fencing. It is primarily impacted by the size and type of electrolysis. As large scale industrial production of electrolysis systems is still in its infancy, Fraunhofer’s IMS assumes that investment costs of electrolysis projects will be roughly cut to 50% of today’s cost level by 2030.
Fixed operational cost (OPEX) is primarily defined by labour and maintenance. Thus, the most probable scenario here are cost increases induced by general inflation effects. Besides, no significant cost changes can be anticipated.
Variable OPEX is dominated by cost of electricity. Cost for water and other consumables which are required in minor quantities can be neglected for an initial estimation of production costs. Renewable electrical power can be generated for 0.03 to 0.17 €/kWh, depending on the utilized energy source and the size of the production facility.
The fourth factor influencing the hydrogen costing system is the utilization of the electrolysis. While variable OPEX is directly proportional to hydrogen production, investment costs and fixed OPEX are constant annual costs. Its impact on cost of production is depending on the overall hydrogen production and thus on capacity utilization. As producers generally try to increase utilization as much as possible, it directly depends on the availability of renewable electricity.
Dependencies in Cost of Production
Cost of production for green hydrogen can, slightly simplified, be calculated from these four factors and the time of depreciation, which is in many cases 10 years. The respective formula is:
CoP = V + (F + I / t) / (U * N)
CoP = cost of production [€/kg]
V = variable OPEX [€/kg]
F = fixed OPEX [€/a]
I = invest [€]
t = time of depreciation [a]
U = utilization [%]
N = nominal Production [kg/a]
As highlighted before, producers can primarily influence two factors: the cost of electricity, i.e. variable OPEX, and capacity utilization. Hydrogen production costs are reduced for lower electricity costs and increasing capacity utilization. Unfortunately, these two factors are not independent of each other for green hydrogen production.
In the example calculations shown in the table below, I assumed a 10 MW electrolysis with 65% efficiency, which would results in an annual hydrogen production of 1,500 t at 100% utilization. The overall investment cost is set at 10 M€ with a 10 years linear depreciation and 150 k€ annual fixed costs. For variable OPEX, only cost of electricity is included while all other costs are neglected for simplicity’s sake.
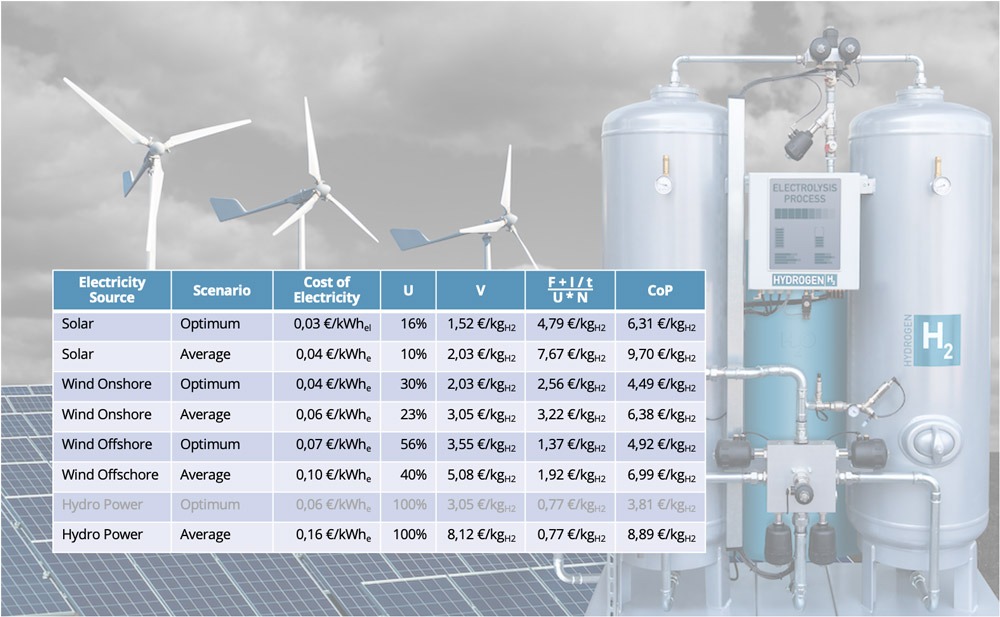
Bearing in mind that potential sites for large-scale hydropower plants are already used in Germany and thus the optimum scenario with a 10 MW hydropower station is unrealistic, the data showcase the reverse trend in variable and fixed elements in production costs.
Optimizing Cost of Production
Looking at the calculated cost of production, the key question is how to produce green hydrogen both ecologically and economically. One important factor will surely be e reduction in hardware cost. Even if a certain fraction of this effect will be balanced by inflation effects, the relative cost of electrolysis units will go down compared to other energy conversion technologies.
While investment costs for electrolysis technology can be expected to decline over time, a similar effect cannot be expected for wind and solar power. Thus, cost reduction for variable OPEX is rather unlikely, especially as sites with optimum wind and solar conditions will soon be utilized and new wind and solar parks will rather be built on B or C sites with lower average power production.

Finally, electrolysis utilization may be increased by use of power storage and a combination of several renewable electricity sources. Batteries or similar electricity storages can significantly increase utilization but are quite expensive as of today. Thus, an optimization of utilization comes at the cost of increased investments and the effect on hydrogen’s production costs are rather negative.
The approach to improve utilization by using several power sources, e.g. wind and solar power, seems to be more promising in reducing lower production costs. This scenario, however, requires an increased power supply which needs to be provided for. This may impact the average cost of electricity and thus has to be evaluated separately.
This example calculation and the discussion of its results highlight the complexity in green hydrogen economics. Contact us or follow us on LinkedIn to learn more about the topic.